Titration Curve of Weak Acid against Strong Base
(image)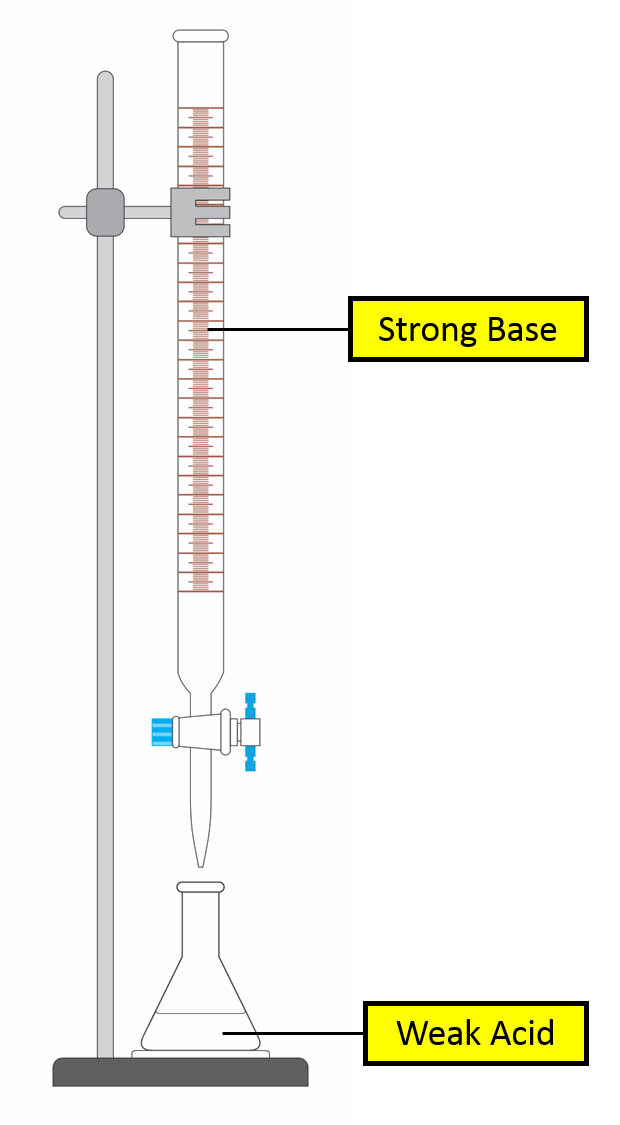
Titration Curve of Weak Acid against Strong Base
(image)
|
Selected Point Burette Volume pHSelect the major species present in the conical flask WA conj base of WA xs SB Is solution a buffer? yes |
|
||||||||||||||||||||||||||||||||||||||||||||